This laboratory session deals with the isolation of pure cultures of microorganisms and the methods used to examine their microscopic morphology. The isolation of pure colonies of microorganisms is essential to the performance of any microbiological procedure. The most commonly employed technique for obtaining pure isolates is the streaking of culture material onto agar plates. This mechanical means of obtaining pure isolates is helped by using special media.
Tryptic Soy Agar (TSA): a general purpose, non-selective, non-differential, supportive medium that supports growth of all microorganisms that do not require special nutrients.
Blood Agar Plate (BAP): a good general purpose, enriched, differential medium that supports the growth of most of the clinically important microorganisms. This medium allows some differentiation of bacterial species based on their ability to lyse the red blood cells contained in the agar.
MacConkey agar (MAC): a selective, differential medium designed to allow growth of only Gram-negative microorganisms, specifically the gram-negative enteric bacilli. This medium allows differentiation of these organisms by their ability or inability to ferment lactose.
Phenyl Ethyl Alcohol Blood agar (PEAB): a selective medium for gram-positive bacteria that retards or completely inhibits the growth of gram-negative organisms. The addition of blood also makes it differential, similar to BAP.
Specimen material is streaked on the surface of these media to yield isolated colonies. Culture specimens may contain many different microorganisms. It is essential for the microbiologist to separate these organisms into distinct, pure colonies before any identification of the organisms can be done.
When plates are streaked for isolation, the organisms present in the inoculum are streaked over the surface of the agar so that individual organisms are dispersed across the plate. Thus each organism is a sufficient distance from all other organisms to allow it to multiply and yield a pure colony of itself. It is assumed that pure colonies result from the division of a single, isolated bacterium.
Aseptic technique refers to procedures by which cultures may be manipulated without infecting the worker or contaminating the cultures or the laboratory environment.
The flaming of lips of tubes and flasks must ALWAYS be done whenever culture liquid is to be poured from a container (e.g., pouring plates). Flaming should be routinely done when caps are removed from tubes during transfer of cultures. The purpose of flaming is not to sterilize, but to warm the tube and create warm air convection currents up and away from the opening. This "umbrella" of warm, rising air will help to prevent the entrance of dust particles upon which contaminating bacteria reside.
Petri dish lids prevent dust from falling directly onto plates but allow diffusion of air around the edges. There are no direct air currents into the plate, and to enter, dust particles would have to rise vertically more than a centimeter. This does not often occur because of the density of the particles. Whenever the lid is removed, it should be held over the plate as a shield. Do not place the lid on the bench top. Do not leave plates uncovered. Do not walk around the room with an open plate.
When working with cultures in testtubes, work as rapidly as is consistent with careful technique. Keep the tubes open a minimum amount of time. While the tubes are open, hold them at a 45 degree angle so that dust cannot fall into the open tube. Hold the tubes away from your face while transferring.
Testtubes are handled in the following manner:
Label all cultures with the name or number of the organism, and your name.
The Gram stain is the most useful stain in microbiology. It allows one to learn both the stain reaction and morphology of bacteria. Almost all decisions regarding procedures used to identify an organism, are made based upon the results of a Gram stain.
The procedure consists of staining with crystal violet (a purple dye), mordanting with iodine, decolorizing
with acetone-alcohol, and counter-staining with safranin (a red dye). Bacteria are divided into two groups
by this procedure. The stain reaction correlates with the type of cell wall makeup.
Those bacteria that are not decolorized retain the purple color of the crystal violet, and are GRAM-
POSITIVE. Those decolorized by the acetone-alcohol are stained red
by the safranin counter-stain and are GRAM-NEGATIVE. 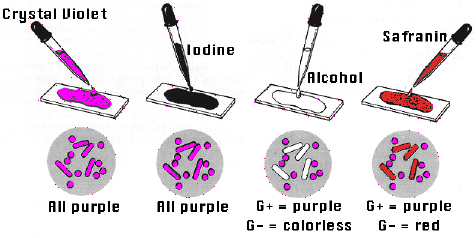
The distinction is not always perfect; dead or dying bacteria stain red whatever their cell wall makeup. Young cultures (18-24 hrs old) should be used for staining. Some bacteria are Gram variable under any condition, but this small group does not significantly detract from the usefulness of this technique.
Preparation of Smears: For any stain to be successful, the smear must be properly prepared. If the smear is too thick, individual cell morphology is obscured; if too thin, the cells will be lost on the vast field of the slide. If properly done, the smear should dry almost immediately, leaving a faint opacity. Many of the difficulties encountered (especially in the decolorization step) are due to improperly prepared smears.
Emulsify your sample taken from one colony with a sterile loop or needle in a very small drop of saline on a clean glass slide. Spread it out to leave a thin film.
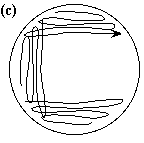
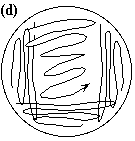
Single Colony Isolation (Quadrant Method) Note: The Single Colony Isolation diagram shows widely spaced streak lines. This is only to illustrate the pattern of the streak lines. The streak lines on a properly streaked plate are very close together, almost touching.
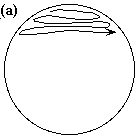 or
inserting the loop into the broth.
or
inserting the loop into the broth.
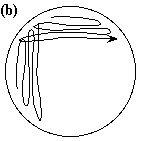 ending by extending the close parallel
strokes into the middle of the plate (b).
ending by extending the close parallel
strokes into the middle of the plate (b).