The exercises in today's and tomorrow's lab will illustrate several types of antigen-antibody reactions.
First: One of the properties antibody classes IgG and IgM have is precipitation from solution when either combines with multivalent antigens -- such reactions can be visualized, and are called precipitation.
Second: Immunoglobulins can be complexed with other molecules such as radioisotopes or enzymes so that we can detect their binding to antigens at extremely low concentrations. Two examples of these sensitive tests are radioimmunoassay or enzyme-immunoassay.
Third: Antibodies can agglutinate multivalent particulate antigens, such as cells or bacteria, and this behavior as an agglutinin can be used to identify (type) such antigens easily and quickly.
Fourth: Antibodies can neutralize the biological activity of a microorganism or its product. Some examples of antibody action are as an opsonin (enhancing phagocytosis), as a reducer of viral infectivity (neutralization), and as an anti-hemolytic factor (against scarlet fever). Such assays can be quantitative, permitting us to determine the quantity of antibody present.
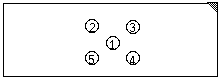
Precipitating antibody is often produced during the normal sequence of events in an infection. This is generally in response to soluble microbial substances released as a consequence of a disintegrative process. The presence of these antibodies can be demonstrated with agar gels wherein antigens and antibodies are allowed to diffuse toward each other. Reaction of antibody with soluble antigen results in a precipitate that will occur at points where equivalent proportions of both antigen and antibody occur. This qualitative technique and others derived from it, is often used to identify antigenic specificities (see demonstration at front table).
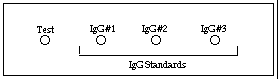
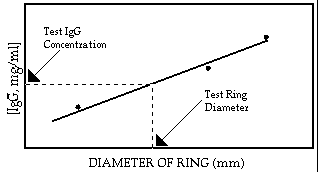
Radial immunodiffusion, a variation of the agar precipitation technique, is used in clinical immunology for the detection and quantitation of all classes of immunoglobulins, complement, ceruplasmin, transferrin, and other serum components. For example, in measuring IgG, antibody to IgG (produced by injecting human IgG into another species) is mixed with agar, poured onto a glass slide and allowed to harden. Known concentrations of IgG (in this case acting as an antigen) are then placed into a series of wells from which they diffuse radially into the antibody containing agar. Since antibody is present throughout the agar, visible circles of precipitate will form around each well that contains IgG. The diameter of the visible ring of precipitate around each well, is proportional to the concentration of the IgG in the well. An unknown sample is also placed in a separate well, and the concentration of IgG in the unknown sample is determined from a standard curve constructed from the ring diameters formed by the IgG standard wells.
The general principle of labeling antibody or antigen in order to follow its subsequent reaction was first applied in the early 1940's. Fluorescent labels were used to follow localization of antibody in tissue sections. The discovery of simple labeling procedures using radioisotopes, especially I125, allowed the use of radioimmunoassays for antigen measurement. Since radioimmunoassay systems offer substantial problems with respect to licensing, disposal and user safety requirements, a variety of tests have been developed which employ enzymes as alternative labels.
In the EMIT (Enzyme Multiplied Immunoassay Technique) system, small molecular weight haptens, e.g., antibiotics, opiates, anticonvulsants, etc., are coupled to the active site of an enzyme. Since they are small, the hapten does not interfere with enzyme activity. However, when antibody is present and bound to hapten in the active site, substrate cannot reach the enzyme's active site and the substrate therefore cannot be broken down. When free hapten is present, e.g., in a serum sample, some of the antibody dissociates from the bound hapten and binds to the free hapten. The result is that the enzyme's active site is open and can now react with substrate. If enzyme-substrate combinations that result in a color change are used, free hapten in the serum can be quantitated on the basis of the amount of color produced.
Antigens and antisera (identified by number)
Gentamicin is an aminoglycoside antibiotic useful in treating patients with Gram-negative bacteremia or severe soft tissue infection. However, the drug has considerable nephrotoxicity and ototoxicity. Consequently, patients should be monitored closely to be sure that free gentamicin levels do not exceed safe levels (about 25 g/ml). The therapeutic range for gentamicin is 5-15 g/ml. This assay illustrates how these levels can be determined.
Materials supplied: (work in pairs)