
EXERCISE 9
ANTIBIOTIC SENSITIVITY TESTING
I. INTRODUCTION
Antibiotics are chemical compounds that selectively interfere with the growth of microorganisms while
causing little or no damage to the animal or human host. Because of occasional adverse side effects and
the increasing emergence of antibiotic resistant organisms, chemotherapeutic agents must be used
properly and judiciously to safeguard their clinical usefulness.
A. Kirby-Bauer Method
The test most widely used for determining the antibiotic(s) to be used to treat an infection is the Kirby-
Bauer method. The suspected pathogen is isolated in pure culture from a clinical specimen and is tested
for its sensitivity to clinically
attainable levels of several antibiotics. The selection of drugs to be used for the test is based primarily on the
knowledge of the infection, and a carefully prepared Gram stain.
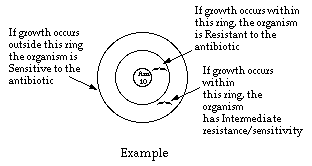
The Kirby-Bauer method is based on the inhibition of bacterial growth measured
under standard conditions. The organism to be tested is grown to a specific turbidity in a standard liquid
medium. An inoculum from this culture is spread across the surface of a Mueller-Hinton agar plate to give
confluent growth. Paper discs containing specific concentrations of antibiotics are placed on the agar surface.
The antibiotic in each disc diffuses outward from the disc, and the concentration of the antibiotic diminishes as
the distance from the disc increases. After incubation, the diameter of the zone of growth inhibition is
measured and scored according to the
size of the zone and the particular antibiotic, as sensitive, intermediate, or resistant.
The size of the zone of inhibition is directly proportional to the sensitivity of the organism to the antibiotic.
Infections due to organisms designated as sensitive to a given antibiotic are more likely to yield clinically to that
antibiotic than are infections with strains designated as intermediate or resistant.
B. MINIMAL INHIBITORY CONCENTRATION (MIC)/
MINIMAL BACTERICIDAL CONCENTRATION (MBC)
Antibiotic sensitivity expressed in terms of the minimal inhibitory concentration (MIC) and/or the
minimal bactericidal concentration (MBC) gives quantitative data not obtainable with the Kirby-
Bauer method. These quantitative results are
useful in predicting the tissue, blood or urine levels of antibiotics that must be attained to assure inhibition
or killing.
The Minimal Inhibitory Concentration (MIC) is determined for each bacterial isolate-antibiotic
combination. Only antibiotics that show inhibitory activity toward the bacterial isolate using the Kirby-
Bauer method are tested further. The active antibiotic is serially diluted to make a range of antibiotic
concentrations that encompasses the concentration used in the Kirby-Bauer method. A constant, small
amount of test bacterium is added to all tubes. After incubation, the MIC is identified as the smallest
concentration of antibiotic that inhibits the growth of the test bacterium. This MIC will inhibit growth but
not necessarily kill. The killing concentration is the MBC.
The Minimal Bactericidal Concentration (MBC) is determined by subculturing each
of the No Growth tubes in the above MIC test to a solid medium that does not
contain antibiotic. The antibiotic in the subculture diffuses into the agar, releasing any inhibitory effect it
may have had on any living bacteria in the subculture. The MBC is identified as the smallest concentration
of antibiotic that prevents any growth of the test bacterium (i.e., kills).
The clinician uses the MIC and MBC data in two ways:
- First, these values, like the zone diameters for Kirby-Bauer, provide a basis
for selecting a drug likely to be effective against the infection.
- Second, MIC and MBC data, more so than the Kirby-Bauer data, can be
useful in judging the dosage of the drug.
Peak drug levels in the blood are planned to exceed the MIC or MBC of the infecting bacterium.
Infections not sufficiently challenged by active host defenses (e.g., in patients with immune deficiency
or endocarditis) require a bactericidal drug at levels above the MBC. Conversely, infections exposed to
an active host defense system often respond to serum levels below the MBC.
Note that the MBC and MIC provide a value at which there is confidence in strong
antibacterial action; there is no need to increase the dosage toward more toxic levels. This balancing of peak
drug level between the MIC and toxicity is most important
for drugs that are toxic at low levels (e.g., aminoglycosides - cf. the EMIT assay in Exercise 2). For such
drugs, drug levels in the patientŐs serum should be monitored to allow for adjustments in the dosage.
II. LAB WORK
A. KIRBY-BAUER METHOD
Materials supplied: (work in pairs)
- 1 BAP of a Gram-negative organism (red dot)
- 1 BAP of a Gram-positive organism (yellow dot)
- 2 Prompt® Inoculation wands
- 2 Prompt® Dilution tubes
- 2 sterile cotton swabs
- 2 Mueller-Hinton agar plates (2 green stripes)
- 1 multi-welled plate containing antibiotic discs
Procedure:
- One student in each pair will test the Gram-positive organism while the
other student will test the Gram-negative organism.
- Prepare the standardized inoculum for the Kirby-Bauer method as follows:
- a.Hold the Prompt® Inoculation wand by the handle and touch the
bottom of the wand to three colonies on the BAP.
- b.Snap off the cap of the Prompt® Dilution tube, insert the wand and
make a tight seal between the wand and the tube.
- c. Vortex on a mixer (one for each side of the room) for at least 5 seconds.
- d. Discard wand in Biohazard bag provided. Replace cap onto tube.
- Moisten a cotton swab in the dilution tube and allow the excess liquid to
flow back into the tube. The swab should be wet but not dripping.
- "Paint" the swab once over the entire surface of the MH agar, as evenly as possible. Turn the
plate 90° and without rewetting the swab, paint the agar again. Discard both swab and dilution
tube in the Biohazard bag.
- Allow the plate to dry for 3-5 minutes. Flame your forceps. Apply antibiotic discs to the agar
surface in a rectangular pattern, keeping them as far apart as possible, but at least 3/4 inch from
the edges of the plate. The chart on page 41 indicates which antibiotic discs should be placed on which bacterial lawn. Gently tap on each disc with the forceps to ensure contact.
Incubate the plates inverted at 37°C for 18-24 hours.
B.MINIMAL INHIBITORY
CONCENTRATION/MINIMAL BACTERICIDAL
CONCENTRATION (MIC/MBC)
Materials supplied: (work in groups of 4)
- 1 rack of 10 numbered tubes containing broth (a completed MIC)
- 1 TSA plate divided into 6 sections (a completed MBC)
Procedure:
- The antibiotic, tetracycline was serially diluted (2-fold dilutions), starting with tube #1 (100 µg/ml) and ending with
tube #9 (tube #10 = Control).
- A constant amount of bacteria was added to all tubes and they were
incubated at 37ˇC for 18-24 hours.
- Mix each tube and examine them for growth, comparing each tube to the Control.
4.The MIC is the lowest concentration of antibiotic that still inhibits growth. Fill out the chart
below.
MIC/MBC Results
| Tube # |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
| TETRACYCLINE CONC. |
|
|
|
|
|
|
|
|
|
Control |
| GROWTH (TUBE) |
|
|
|
|
|
|
|
|
|
|
| GROWTH (PLATE) |
|
|
|
|
|
|
|
|
|
|
THE MIC OF TETRACYCLINE FOR THIS STRAIN OF E. coli
IS _____ug/ml.
- From each of the NEGATIVE tubes (tubes in which no growth is apparent)
and the Control tube, samples were swabbed onto the surface of a TSA plate.
- The plate was incubated at 37ˇC for 18-24 hours.
- The MBC is the least concentration of antibiotic that does not permit growth.
THE MBC OF TETRACYCLINE FOR THIS STRAIN OF E. coli
IS _____ug/ml.
With which kind of antibiotic (static or cidal) would you expect the MIC and MBC
to be about the same concentration? To be vastly Different concentrations?
NEXT LAB PERIOD
- Compare growth inhibition zones on the Mueller-Hinton plate with the
appropriate inhibition patterns on the next page.
- Record the extent of growth inhibition as R (resistant), I (intermediate), or S
(sensitive) for each antibiotic.
- Sample results
- Measuring the tetracycline inhibition zone
Kirby-Bauer Sensitivity Results
| GRAM POSITIVE |
GRAM NEGATIVE |
| Antibiotic | Results | Antibiotic | Results |
| Ampicillin |
| Ampicillin |
|
| Erythromycin |
| Nalidixic Acid |
|
| Penicillin |
| Streptomycin |
|
| Tetracycline |
| Tetracycline |
|
While every effort is made to ensure that this information is up-to-date and accurate, the statements found on this page are for informational purposes only.LSUMC WWW Publication Policy, 9/23/96



