
Chu Chen, Ph.D.
Chu Chen, Ph.D.
Professor of Neuroscience and Otorhinolaryngology
Professor of Neuroscience and Otorhinolaryngology
2020 Gravier Street, Suite D
New Orleans, LA 70112
Phone: (504) 568-8458
Fax: (504) 599-0488
cchen@lsuhsc.edu
Degrees
Education
1994-1996 Postdoctoral fellow, LSU Health Sciences Center, New Orleans
1989-1993 PhD, Tulane University, New Orleans
1983-1986 MS, Zhejiang Medical University, Hangzhou, China
1979-1983 BS, Nanjing University, Nanjing, China
Academic positions
7/2013 – present
Professor with tenure, Neuroscience Center and Department of Otorhinolaryngology, LSU Health Sciences Center in New Orleans
7/2008 - 6/2013
Associate Professor with tenure, Neuroscience Center and Department of Otorhinolaryngology, LSU Health Sciences Center in New Orleans
2002-2008
Tenure track Assistant Professor, Department of Otorhinolaryngology and Neuroscience Center of Excellence, LSUHSC
1998-2002
Research Assistant Professor, Department of Otorhinolaryngology and Neuroscience Center of Excellence, LSUHSC
1996-1998
Research Assistant Professor, Department of Otorhinolaryngology, LSUHSC
1988-1989
Lecturer, Department of Physiology, Zhejiang Medical University, Hangzhou, China
1986-1988
Instructor, Department of Physiology, Zhejiang Medical University, Hangzhou, China
Bio
Dr. Chu Chen
2016 Distinguised Alumnus Award
Tulane University 1993
2015 Scientific Innovation Award, LSU Health Sciences Center
Study Sections
- Alzheimer’s Association grants (2004-2014)
- National Science Foundation Grants
- Irish Health Research Board grants, Ireland (2005-2006)
- Indiana University Alzheimer’s Disease Research Grants (2008-2009)
- Wellcome Trust Research grants, UK (2010)
- NIH NIDA Special Emphasis Panel ZDA1 - JXR – D (06) (2011)
-
NIH CSR Special Emphasis Panel ZRG1 MDCN-R (40) P- 2014
- Medical Research Council (MRC), UK (2012)
- Florida State the Ed and Ethel Moore Alzheimer’s Disease Research Program Review Panel
- NIH NIGMS special emphasis panel ZGM1 RCB-6 (C2) - 2017
Research Interests
Keywords:
Neuroinflammation in health and disease;
Molecular and epigenetic mechanisms of neurodegenerative diseases, including
Alzheimer’s disease and traumatic brain injury (TBI)-induced AD-like neuropathology;
Endocannabinoid signaling in neuroinflammation, neuroprotection, synaptic plasticity, learning and memory;
Mechanisms of marijuana-altered synaptic and cognitive function, and cannabinoid tolerance.
Research Interests
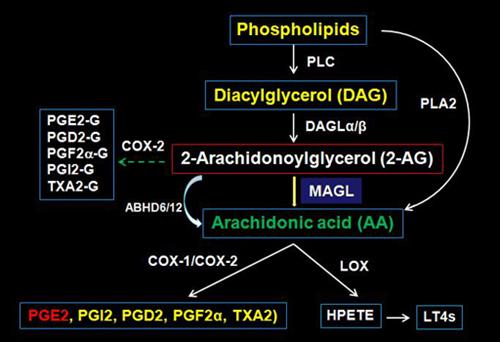
Inflammation is now believed to be a common mechanism of disease. Neuroinflammation, the inflammatory response that occurs in the nervous system, has been implicated in many brain disorders, including epilepsy, traumatic brain injury, stroke, and neurodegenerative diseases such as multiple sclerosis, Parkinson’s and Alzheimer’s diseases. However, our understanding of the molecular mechanisms underlying neuroinflammation in the pathogeneses of neurodegenerative diseases is still limited. My research programs focus on neuroinflammation in synaptic plasticity and neurodegenerative diseases. Specifically, I am interested in endocannabinoid and prostaglandin signaling in hippocampal synaptic plasticity and pathogenesis of Alzheimer’s disease. Recent evidence shows that a large proportion of prostaglandins derives from hydrolysis of the endogenous cannabinoid 2-arachidonoylglycerol (2-AG) by monoacylglycerol lipase (MAGL), the enzyme that primarily metabolizes 2-AG in the brain. While 2-AG exhibits anti-inflammatory and neuroprotective properties, prostaglandins (e.g., PGE2) are proinflammatory and neurotoxic. Therefore, MAGL plays an important role in homeostatic regulation of endocannabinoid 2-AG and prostaglandin signaling (See inset) in physiology and diseases. Thus, inhibition of MAGL will result in strengthening anti-inflammatory and neuroprotective 2-AG signaling, while reducing proinflammatory arachidonic acid and prostaglandin levels. We are currently addressing this important issue as to whether inactivation of MAGL is able to impromve hippocampal synaptic plasticity, learning and memory and reduce neuropathology of Alzheimer’s disease and whether epigenetic mechanisms such as noncoding small RNAs (miRNAs) are involved in regulation of synaptic plasticity and neuropathology by endocannabinoid and prostaglandin signaling.
Teaching Activities
2002-present Lecture: Investigative Neuroscience 203 to PhD graduate students
2002-present Lecture: Molecular Neurobiology 250 to PhD graduate students
Selected Publications
-
Zhang J and Chen C. (2018) Alleviation of Neuropathology by Inhibition of Monoacylglycerol Lipase in APP Transgenic Mice Lacking CB2 Receptors. Molecular Neurobiology 55: 4802-4810.
- Zhang J and Chen C. (2017) Δ9-THC and COX-2 signaling. Chapter 75, in Victor R Preedy (Ed), Handbook of Cannabis and Related Pathologies. Elsevier Publisher, Amsterdam, Netherlands, ISBN: B978-0-12-800756-3; pp: 729-738.
-
Chen C. (2016) Endocannabinoid metabolism in neurodegenerative diseases. Neuroimmunology and Neuroinflammation. 3:268-270.
(Editorial)
-
Chen C. (2015) Homeostatic regulation of brain functions by endocannabinoid signaling. Neural Regeneration Research 10:691-692.
- Song Y, Zhang J & Chen C. (2015) Fine-tuning of synaptic upscaling at excitatory synapses by endocannabinoid signaling is mediated via the CB1 receptor. Scientific Reports 5: 16257.
- Zhang J, Teng Z, Song Y, Hu M & Chen C. (2015) Inhibition of monoacylglycerol lipase prevents chronic traumatic encephalopathy-like neuropathology in a mouse model of repetitive mild closed head injury. Journal of Cerebral Blood Flow & Metabolism 35:443-453.
-
Xu J & Chen C. (2015)
Endocannabinoids in synaptic plasticity and neuroprotection. Neuroscientist 21:152-168.
- Zhang J, Hu M, Teng Z, Tang Y & Chen C. (2014) Synaptic and cognitive improvements by inhibition of 2-AG metabolism are through upregulation of microRNA-188-3p in a mouse model of Alzheimer’s disease. Journal of Neuroscience 34:14919-14933. (Featured article). Highlighted in Nature-Science-Business eXchange: http://www.nature.com/scibx/journal/v7/n46/full/scibx.2014.1354.html
- Chen R, Zhang J, Fan N, Teng Z, Wu Y, Yang H, Tang Y, Sun H, Song Y & Chen C. (2013) Δ9-THC-caused synaptic and memory impairments are mediated through COX-2 signaling. Cell 155:1154-1165. (Featured and highlighted in Cell:http://www.elsevier.com/connect/preventing-marijuana-induced-memory-problems-with-OTC-painkillers;Science: http://news.sciencemag.org/brain-behavior/2013/11/painkillers-may-curb-memory-loss-medical-marijuana; Science Signaling: Snuffing Out Marijuana Inflammation. 6(304):ec291,2013: http://stke.sciencemag.org/cgi/content/abstract/6/304/ec291; Nature: Painkiller kills the bad effects of pot. 503(7477):440,2013: http://www.nature.com/nature/journal/v503/n7477/full/503440b.html; Nature Reviews Neuroscience: Memory: a boost for medical marijuana? 15(1): 2-3,2014: http://www.nature.com/nrn/journal/v15/n1/full/nrn3659.html; and covered by various news media)
- Chen R, Zhang J, Wu Y, Wang D, Feng G, Tang YP, Teng Z & Chen C. (2012) Monoacylglycerol lipase is a therapeutic target for Alzheimer’s disease. Cell Reports 2:1329-1339 (Covered by various news media).
- He H, Mahnke A, Doyle S, Fan N, Wang C, Hall B, Tang YP, Inglis F, Chen C & Erickson J.D. (2012) A neurodevelopmental role for VGLUT2 in pyramidal neuron maturation and spatial learning. Journal of Neuroscience 32:15886-15901
- Xu J, Zhang J & Chen C., (2012) Long-lasting potentiation of hippocampal synaptic transmission by direct cortical input is mediated via endocannabinoids. Journal of Physiology (Lond) 590:2305-2315.
- Du H, Chen X, Zhang J & Chen C. (2011) Inhibition of COX-2 expression by endocannabinoid 2-arachidonoylglycerol is mediated by PPAR-gamma. British Journal of Pharmacology (2011) 163:1533-1549.
- Chen X, Zhang J & Chen C. (2011) Endocannabinoid 2-arachidonoylglycerol protects neurons against beta-amyloid insults. Neuroscience 178:159-168.
- Yu T, Li Z, Jia Z, Clapcote SJ, Liu C, Li S, Asrar S, Pao A, Chen R, Fan N, Carattini-Rivera S, Bechard AR, Spring S, Henkelman RM, Stoica G, Matsui S-I, Nowak NJ, Roder JC, Chen C, Bradley A & Yu YE. (2010) A mouse model of Down Syndrome trisomic for all human chromosome 21 syntenic regions. Human Molecular Genetics 19:2780–2791.
-
Chen C. (2010) COX-2’s new role in inflammation. Nature Chemical Biology 6: 401-402.
- Xu J, Zhang J & Chen C. (2010) Endocannabinoids differentially modulate long-term depression in rat hippocampal CA1 pyramidal neurons. PLoS ONE 5:e10306.
- Fan N, Yang H, Zhang J & Chen C. (2010) Reduced expression of glutamate receptors and phosphorylation of CREB are responsible for Δ9-THC-impaired hippocampal synaptic plasticity. Journal of Neurochemistry 112:691-702.
- Grewal S, Defamie N, Zhang X, Gois SD, Shawki A, Mackenzie B, Chen C, Varoqui H & Erickson J.D. (2009) SNAT2 amino-acid transporter is regulated by osmolytes of the SLC6 GABA transporter subfamily and may play no role in delivering glutamine for spontaneous glutamatergic transmission. Journal of Biological Chemistry 284:11224-11236.
- Yang H, Zhang J, Breyer RM & Chen C. (2009) Altered hippocampal long-term synaptic plasticity in mice deficient in the PGE2 EP2 receptor. Journal of Neurochemistry 108: 295-304.
- Zhang J & Chen C. (2008) Endocannabinoid 2-arachidonoylglycerol protects neurons by limiting COX-2 elevation. Journal of Biological Chemistry 283: 22601–22611.
- Yang H & Chen C. (2008) COX-2 in synaptic signaling. Current Pharmaceutical Design 14: 1443-1451.
- Yang H, Zhang J, Andreasson K & Chen C. (2008)COX-2 Oxidative metabolism of endocannabinoids augments hippocampal synaptic plasticity. Molecular and Cellular Neuroscience 37: 682-695.
- Sang N, Zhang J & Chen C. (2007) COX-2 oxidative metabolite of endocannabinoid 2-AG enhances excitatory glutamatergic synaptic transmission and induces neurotoxicity. Journal of Neurochemistry 102: 1966-1977.
- Sang N & Chen C. (2006) Lipid signaling and synaptic plasticity. Neuroscientist 12: 425-434.
- Sang N, Zhang J & Chen C. (2006) PGE2 glycerol ester, a COX-2 oxidative metabolite of 2-arachidonoyl glycerol, modulates inhibitory synaptic transmission in mouse hippocampal neurons. Journal of Physiology (Lond) 572: 735-745.
- Sang N, Zhang J, Marcheselli V, Bazan, NG & Chen C. (2005) Postsynaptically synthesized prostaglandin E2 modulates hippocampal synaptic transmission via a presynaptic PGE2 EP2 receptor. Journal of Neuroscience 25: 9858-9870.
- Gois SD, Schafer MK-H, Defamie N, Chen C, Ricci A, Weihe E, Varoqui H & Erickson JD. (2005) Homeostatic scaling of vesicular glutamate and GABA transporters expression in rat neocortical circuits. Journal of Neuroscience 25:7121-7133.
-
Chen C & Bazan N.G. (2005) Endogenous PGE2 regulates membrane excitability and synaptic transmission in rat hippocampal CA1 pyramidal neurons. Journal of Neurophysiology 93: 929-941.
-
Chen C. (2004) ZD7288 inhibits postsynaptic glutamate receptor-mediated responses at hippocampal perforant path-granule cells synapses. European Journal of Neuroscience 19: 643-649.
- McDermott CM, LaHoste GL, Chen C, Musto A, Bazan NG & Magee JC. (2003) Sleep deprivation causes behavioral, synaptic and membrane excitability alterations in hippocampal neurons. Journal of Neuroscience 23: 9687-9695.
-
Chen C, Magee JC & Bazan NG, (2002) Cyclooxygenase-2 regulates prostaglandin E2 signaling in hippocampal long-term synaptic plasticity. Journal of Neurophysiology 87:2851-2857.
- Rodriguez de Turco EBR, Tang W, Topham MK, Sakane F, Marcheselli VL, Chen C, Taketomi A, Prescott S & Bazan NG. (2001) Diacylglcerol kinase epsilon regulates suizure susceptibility and long-term potentiation through arachidonoyl-inositol lipid signaling. Proceedings of National Academy of Science USA 98: 4740-4745.
-
Chen C., Magee J.C., Marcheselli V., Hardy M. & Bazan N.G. (2001) Attenuated long-term potentiation in hippocampal dentate gyrus neurons of mice deficient in the PAF receptor. Journal of Neurophysiology 85: 384-390.
Additional Info
Current Funding
Source: NIH-NINDS R01NS076815 6/15/2012-4/30/2023
Title: Endocannabinoids in Neurodegenerative Diseases
Role on Grant: PI
Source: NIH-NIMH R01MH113535 2/5/2018-11/30/2022
Title: Endocannabinoid Metabolism and Synaptic Function
Role on Grant: PI
Source: NIH-NIA R01AG058621-01 6/1/2018-2/28/2023
Title: Traumatic Brain Injury and Alzheimer's disease
Role on Grant: PI

 myLSUHSC
myLSUHSC