
Youming Lu, M.D., Ph.D.
Youming Lu, M.D., Ph.D.
Professor of Neurology and Neuroscience
Bollinger Professor of Alzheimer’s Disease
Professor of Neurology and Neuroscience
Bollinger Professor of Alzheimer’s Disease
Neuroscience Center of Excellence FAX: 599-0832
2020 Gravier Street
New Orleans, LA 70112
Office: 599-0874
ylu1@lsuhsc.edu
Degrees
1996-1999: Ph.D., University of Toronto, Canada
1993-1995: Postdoctoral Fellow, University of California, Irvine
1990-1993: Postdoctoral Fellow, Novartis, Basal, Switzerland
1987-1990: M.S., China Pharmaceutical University, China
Bio
2008- Professor, LSU School of Medicine, New Orleans
2004-2008: Associate Professor, University of Central Florida, Orlando
2000-2004: Assistant Professor, University of Calgary, Calgary
Awards and Recognitions
2000-2006: New Investigator Award by Canada Institute for Health Research
2000-2005: Research Scholar Award by Alberta Heritage Foundation for Medical Research
1999-2001: Senior Research Fellowship by Canada Institute for Health Research
1997-1999: Tenable Merit Scholarship by University of Toronto
1997-1999: Laidlaw First Award by University of Toronto
1998-1999: Siminovitch Outstanding Research Award by University of Toronto
1998: Joe A. Connolly Memorial Outstanding Research Award by University of Toronto
1996-1999: Doctoral Research Award by Medical Research Council of Canada
1991-1993: Postdoctoral Fellow by Ciba Foundation, Switzerland
Research Interests
RESEARCH INTERESTS AND GOAL
I am interested in identifying and characterizing the specific cellular and molecular signaling processes that cause the aberrant changes of synaptic transmission and in applying this knowledge for developing the practical strategies in the treatment of Alzheimer’s diseases and stroke.
CURRENT RESEARCH
Glutamate is the major excitatory transmitter in the mammalian central nervous system (CNS) and plays an essential role in neural development, excitatory synaptic transmission, and plasticity. In case of brain injury such as stroke, however, glutamate accumulates at synapses, resulting in extensive stimulation of its receptors that can eventually be toxic to neurons. Although excess stimulation of glutamate transmission contributes to neuronal death, blocking it totally could be deleterious to animals and humans because targeting glutamate synapses would block the synaptic physiological function as well. My long-term objective is to explore an ideal approach for treatment of some neurological disorders and brain injury by targeting at the specific glutamate “cell death signals” whereby the pathological effects of glutamate transmission is selectively blocked, leaving the physiological action unaffected. To achieve this goal, I am currently investigating the specific cellular and molecular signaling processes that cause the aberrant changes of glutamate transmission with two independent research projects, as summarized in the following working model:
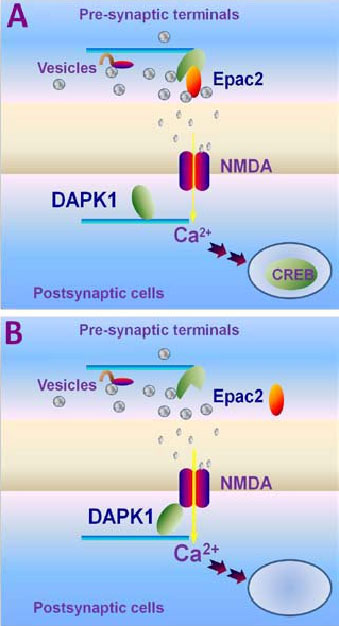
Cell death signals induce the
aberrant changes of synaptic transmission
(A) Under the physiological conditions, excitatory neurotransmitter glutamate releases from the pre-synaptic terminals through synaptic vesicle fusion events, in which Epac, exchange factor that directly activated by cAMP, is involved. Released glutamate binds to its receptors on the post-synaptic sites. One of the principle glutamate receptors is NMDA receptor. Activation of NMDA receptor allows physiological Ca2+ influx and in turn induces gene expression, neuronal growth, synaptic transmission and plasticity. (B) Research in my laboratory has mainly focused on the aberrant changes of synaptic transmission in brain disorders and injury. One of our research projects is targeting to the pre-synaptic terminals, in which Epac mutation deteriorates glutamate release event. We have recently generated the mutant mice with deficiency in expression of Epac genes. We found that genetic deletion of Epac gene declines excitatory synaptic transmission. The second project is focusing on the post-synaptic glutamate receptors. We have discovered that ischemia recruits DAPK1, death-associated protein kinase 1, into the NMDA receptor complex, and induces toxic Ca2+ influx through the receptor channels. We have thus generated the null mutant mice lacking the DAPK1 gene and found that genetic deletion of DAPK1 protects against brain cells death.
Selected Publications
Key Papers
Tu, W., Xu, X., Peng, L., Zhong X., Zhang, W., Soundarapandian, M.M., Belal, C., Wang, M., Jia, N., Lew, F., Chan, S.L., Chen, Y., Lu, Y:DAPK1 interaction with NMDA receptor NR2B subunits mediates brain damage in stroke. Cell (2010) 140:222-234.
Kim, D., Frank, C.L., Dobbin, M.M., W. Tu., Peng, L.S., Lee, B-H., Giusti, P., Broodie, N., Mazitschek, R., Lu, Y. Tsai L-H: Deregulation of HDAC1 by p25/cdk5 in neurotoxicity. Neuron (2008) 60:801-817.
Peng, P.L., Zhong, X.F., Tu, W.H., Soundarapandian, M.M., Molner, P., Zhu, D.Y., Liu, S.H., Lu, Y: ADAR2-Dependent RNA Editing of AMPA Receptor Subunit GluR2 Determines Vulnerability of Neurons in Forebrain Ischemia. Neuron (2006) 49:719-733.
Liu, S.H., Lau, L., Wei, J.S., Zhu, D.Y., Zou, S., Sun, H.S., Fu, Y.P., Liu, F., Lu, Y: Expression of Ca2+-permeable AMPA Receptor Channels Primes Cell Death in Transient
Forebrain Ischemia. Neuron (2004) 43:43-55.
Zhu, D.Y., Lau, L., Liu, S.H,. Wei, J.S., Lu, Y: Activation of cAMP response element binding protein (CREB) after focal cerebral ischemia stimulates neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. USA. (2004) 101:9453-9457.
Wang, J., Liu, S.H., Fu, Y.P., Wang, J.H., Lu, Y: Cdk5 activation induces CA1 pyramidal cell death by direct phosphorylating NMDA receptors. Nature Neuroscience (2003) 6:1039-1047.
Lu, Y., Mansuy, I.M., Kandel, E.R., Roder, J: Calcineurin-mediated LTD of GABAergic inhibition underlies the increased excitability of CA1 neurons associated with LTP. Neuron (2000) 26:197-205.
Lu, Y., Roder, J., Davidow, J., Salter, M.W: Src activation in the induction of long-term potentiation in CA1 hippocampal neurons. Science (1998) 279:1363-1967.
Additional Info
ACTIVE FUNDING
ADAR2-dependent RNA editing of AMPA receptor subunit GluR2 in ischemia
R01 NS051383 (PI: Lu)
03/2006 – 02/2011
NIH/NINDS
DAPK1 regulation of NMDA receptor in ischemic neuronal death
R01 AG033282 (PI: Lu)
08/2008 - 07/2013
NIH/NIA

 myLSUHSC
myLSUHSC