
Walter J. Lukiw, BS, MS, PhD
Walter J. Lukiw, BS, MS, PhD
Professor of Neuroscience, Neurology and Ophthalmology
Bollinger Professor of Alzheimer's disease (AD)
Professor of Neuroscience, Neurology and Ophthalmology
Bollinger Professor of Alzheimer's disease (AD)
Neuroscience Center of Excellence
School of Medicine, LSU Health
2020 Gravier Street, Room 904
New Orleans, LA 70112
Phone: (504) 599-0842
Fax: (504) 568-5801
wlukiw@lsuhsc.edu
Degrees
1986: M.S. 'Gene expression in neurodegenerative disease' - York University & Institute for Basic Research in Developmental Disabilities, Staten Island, NY, USA
1992: Ph.D. 'Gene expression and genotoxicity in Alzheimer's disease' - Neuroscience & Molecular Biology, University of Toronto, Toronto ON, CANADA
Bio
Special Research Recognition (last 16 invited Speaking Engagements)
2018 - invited platform speaker, ‘Secretory products of the human GI tract microbiome and their potential impact on Alzheimer's disease (AD): detection of lipopolysaccharide (LPS) in AD hippocampus and temporal lobe neocortex’ AD-PD 2018 Congress, Torino ITALY.
2018 - invited symposium speaker, ‘NF-kB-upregulated pathogenic microRNAs (miRNAs) in age-related macular degeneration (AMD) and Alzheimer's disease (AD) contribute interactively to altered innate-immune signaling, amyloidogenesis, and inflammatory neurodegeneration’ ARVO annual meeting, Honolulu HI, USA.
2018 - invited speaker platform, Synergism in aluminum and mercury neurotoxicity AutismOne 2018 Meeting 22-27 May 2018 Lombard IL, USA.
2018- invited speaker, ‘Microbial lipopolysaccharides inAlzheimer’s disease (AD) brain’, Moscow State University, Vavilov Institute of General Genetics; Russian Academy of Medical Sciences, Moscow RUSSIA.
2018 – invited speaker – ‘microRNAs (miRNAs) In Alzheimer's disease (AD) and Age-related Macular Degeneration (AMD) Contribute Interactively to Amyloidogenesis, Inflammatory Neurodegeneration, and Altered Innate-immune Signaling’ American Neurological Association (ANA) 143rd Annual Meeting 21-23 October 2018; Atlanta GA, USA.
2018 - invited speaker – ‘Microbiome-derived Bacteroides fragilis Lipopolysaccharide-mediated Up-regulation of NF-kB(p50/p65) and microRNA-146a In Sporadic Alzheimer’s Disease (AD)’ American Neurological Association (ANA) 143rd Annual Meeting 21-23 October 2018; Atlanta GA, USA.
2018 – presenter - Microbiome-derived lipopolysaccharides (LPS) induce NF-kB and microRNA signaling in Alzheimer’s disease (AD); selective knockout of neurofilament light chain (NF-L) gene expression, Society for Neuroscience, San Diego CA, USA.
2019 – invited symposium speaker, ‘Aluminum in neurological and neurodegenerative disease;
a tribute to the work of the late DRC McLachlan BS, MS, OC’; 13th Annual Keele Meeting on Aluminum, Uxmal, MEXICO
2019 - invited platform speaker, ‘Gastrointestinal (GI) tract-derived Bacteroidetes-fragilis neurotoxins and inflammatory neurodegeneration’ Mossakowski Medical Centre, Polish Academy of Medicine, Katowice POLAND.
2019 - invited platform speaker, 'Human GI-tract microbiome-derived Bacteroides fragilis lipopolysaccharide (BF-LPS) up-regulates a pro-Inflammatory miRNA-146a in Alzheimer's disease (AD) brain’ Society for Neuroscience (SFN) 2019 Annual Meeting, Chicago IL, USA.
2020 - accepted abstract;‘Pathogenetic signaling pathways in age-related macular degeneration (AMD), Alzheimer's disease (AD) and prion disease (PrD) involve the participation of common pro-inflammatory, and innate-immune disrupting microRNAs that include miRNA-34a, miRNA-146a and miRNA-155’; ARVO Annual Meeting Baltimore MD, USA (in person meeting cancelled due to CoV-19 and presented online).
2020 - Invited platform speaker ‘Aluminum-stimulated production of lipopolysaccharide (LPS) from the human gastrointestinal (GI)-tract microbiome-resident Bacteroides fragilis’ - International Conference on Cellular & Mol. Biology Rome, ITALY (in person meeting cancelled due to CoV-19 and presented online).
2020 - invited speaker, ‘Environmentally abundant neurotoxins, microRNAs (miRNAs) and inflammatory neurodegenerative disease’ - Environmental Mutagenesis/Genetics (EMGS) Annual meeting, Palm Springs CA, USA; (in person meeting cancelled due to CoV-19 pandemic and presented via Skype online).
2020 – invited speaker, ‘Neuroinflammation the gastrointestinal (GI) tract microbiome and microRNA signaling in the brain and retina’ Alzheimer’s Association International Congress (AAIC), Amsterdam NETHERLANDS; (in person meeting cancelled due to the CoV-19 pandemic and presented online via Zoom).
2020 - invited platform speaker, ’Human gastrointestinal (GI) tract microbiome-derived neurotoxins - contribution to inflammatory neurodegeneration in Alzheimer’s disease (AD) brain, American Neurological Society Annual Meeting Los Angeles CA, USA; (in person meeting cancelled due to CoV-19 and presented online)
September, 2013 – invited platform speaker ‘MicroRNA (miRNA); sequence and stability, viroid-like properties, and disease transmission in the central nervous system (CNS)’, Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, POLAND.
October, 2013 – invited platform speaker ‘Monoamine oxidase inhibitors (MAOIs) block ocular pathology, HSV-1 replication, and neuronal reactivation in the rabbit eye model’, Asia-ARVO; 28-31 October 2013, Ashok International Convention and Exhibition Centre, New Delhi, INDIA.
October, 2013 – invited platform speaker – ‘Amyloid peptide (Aβ42)-mediated inflammatory signaling in the uveitis and retina – relevance to ocular inflammation and innate immune signaling’, Asia-ARVO; 28-31 October 2013, Ashok International Convention and Exhibition Centre, New Delhi, INDIA.
November, 2013 – invited symposium presentation – ‘Regulation of TREM2 expression by an inducible, NF-kB-sensitive miRNA-34a’, Society for Neuroscience (SFN), San Diego CA, USA.
March, 2014 – invited platform speaker –‘Aluminum accumulation within the human central nervous system (CNS) – targeted genetic and epigenetic effects on innate-immunity, phagocytosis and CNS-specific inflammation’, 9th International Congress on Autoimmunity, 24-30 March 2014 Nice, FRANCE.
May, 2014 – invited platform presentation – ‘Micro RNA (miRNA) complexity in the extracellular fluid (ECF) and cerebrospinal fluid (CSF) of Alzheimer’s disease (AD) and related neurodegenerative disorders’, Biomarkers & Diagnostics World Congress April 30-May 2, 2014, Philadelphia PA, USA.
July, 2014 – invited seminar speaker – 'Complement factor H (CFH) and the triggering receptor expressed in microglial cells (TREM2): common regulation by microRNAs (miRNAs) in Alzheimer's disease (AD) and age-related macular degeneration (AMD)'; Departments of Neuroscience and Pharmacology, Panum Institute, University of Copenhagen, July 15, 2014, Copenhagen, DENMARK.
October, 2014 – invited platform speaker – 'Studies on non-coding RNA (ncRNA) and messenger RNA (mRNA) complexity in Alzheimer’s disease (AD) and the aging human brain', at the opening of the new Neuroscience Research Center of Indiana University, IUPUI, October 9, 2014, Indianapolis IN, USA.
November, 2014 – invited special symposium speaker – ‘Circular RNA (circRNA) ciRS-7 in Alzheimer's disease (AD) mediates microRNA-7 (miRNA-7) trafficking', Society for Neuroscience Annual Meeting 15-19, November 2014, Washington DC, USA.
Current Research Projects:
(1) Genetic regulatory mechanisms in growth and invasiveness of glioblastoma multiforme (GBM)
(2) Metal sulfate-mediated oxidative stress and pro-inflammatory gene signaling in human brain cell models of Alzheimer's disease (AD)
(3) Inflammatory signaling and gene expression in Alzheimer's disease (AD)
(4) microRNA (miRNA) signaling in Alzheimer's disease (AD) and transgenic AD (Tg-AD) models
Current Research:
Molecular-genetic mechanisms involved in pathological signaling in age-related macular degeneration (AMD), Alzheimer’s disease (AD), glioblastoma multiforme (GBM); potential drug strategies for the clinical improvement of these neurological disorders.
Editorial Boards
- 2003 -– ‘Cellular & Molecular Neurobiology’, Associate Editor, Plenum Publishers, Dordrecht, HOLLAND
- 2003 -– ‘Neurochemical Research’, Associate Editor, Kluwer Academic/Plenum Publishers, NY, USA
- 2005 -– ‘Molecular Neurobiology’, Associate Editor, Editorial Board, Humana Press, NJ, USA
- 2005-2006 -– ‘Journal of Alzheimer’s disease’, Associate editor (one year appointment), IOS Press, Amsterdam, HOLLAND
- 2009 –- ‘Frontiers in Non-Coding RNA’, Associate Editor, Frontiers Press, Lausanne, SWITZERLAND (Note: ‘Frontiers’ current monthly readership is currently over 3 million in 110 countries)
- 2010 -– ‘International Journal of Biochemistry & Molecular Biology’, Senior Editorial Board, Madison WI, USA
- 2011 -– ‘PLoS ONE’, Public Library of Science, Academic Editor, Senior Editorial Board, San Francisco CA, USA and London, ENGLAND
- 2011 -– ‘Folia Neuropathologica’, Editorial Board, (official journal of the Polish Academy of Medical Sciences and the Polish Association of Neuropathologists), Warsaw, POLAND
- 2011 -– ‘Expert Opinion on Emerging Drugs’, Senior Editorial Board Member, Los Angeles CA, USA
- 2011 -– ‘American Journal of Alzheimer’s Disease’, Associate Editor, Columbia MO, USA
- 2011 -– ‘Outlook on Developing Drugs: Open Access’, OMICS Publishing Group, Los Angeles CA, USA
- 2012 -– ‘The American Journal of Neurodegenerative Disease (AJND)’, Jacksonville, FL, USA
Study Sections
- Pfizer-FRQS Innovation Fund for Alzheimer's Disease and Related Disorders, Montreal QC, CANADA
- Alberta Heritage Foundation for Scientific & Medical Research, Edmonton AB, CANADA
- Alzheimer’s Society of Canada, Toronto ON, CANADA
- Alzheimer’s Association, Chicago IL, USA
- American Institute of Biological Sciences, (AIBS) Reston VA, USA
- Canadian Liver Foundation, Toronto ON, CANADA
- European Research Grant Directive LECMA-AFI-ISAO, Paris, FRANCE
- Feralex Corporation, Toronto ON, CANADA
- Israeli Science Foundation, (ISF) Rehovot/Jerusalem, ISRAEL
- Militarily Relevant Peer-Reviewed Alzheimer’s Disease Research Program (MRPRA), Reston VA, USA
- National Institutes of Health – NIA study sections 2007-2012; by email and at Bethesda MD, USA
- National Institutes of Mental Health – NIMH email reviewer, Bethesda MD, USA
- National Medical Research Council (MRC) of Singapore, SINGAPORE
- NMRC International Peer Review Expert Study Section, SINGAPORE
- Science Foundation Ireland (SFI); Dublin, IRELAND
- Research Advisory Council of the Ontario Workplace Safety and Insurance Board (WSIB-RAC)Institute, Toronto ON, CANADA
- Stichting voor Alzheimer Onderzoek, Fondation pour la Recherche sur la Maladie d’Alzheimer, FRMA Scientific Secretariat, University of Antwerp- CDE, Antwerp, BELGIUM
- Scientific Evaluation Committee, French National Research Agency (FNRA-ANR), Paris, FRANCE

Dr. Susumu Tonegawa (Nobel Laureate in Medicine 1987, left) and Dr. Walter J. Lukiw (LSUHSC Neuroscience Center of Excellence, right) May 2008 2020 - invited platform speaker, ‘At the intersection of neurotoxins, microRNAs (miRNAs) and inflammatory neurodegenerative disease’ Society for Neuroscience (SFN) Annual Meeting, Washington DC, USA.Special Research Recognition (last 10 invited Speaking Engagements)October, 2013 – invited symposium speaker ‘Common mechanisms forTREM2 (chr6p21.1) and CFH (chr1q32) regulation by NF-kB-sensitive miRNAs in age-related macular degeneration (AMD) and Alzheimer's disease (AD)’, Asia-ARVO; 28-31 October 2013, Ashok International Convention and Exhibition Centre, New Delhi, INDIA.
Research Interests
Keywords:
Alzheimer's disease, bioinformatics, brain-specific transcription, changes in the mammalian brain associated with aging, DNA arrays, gene expression analysis and profiling, memory, neurotoxicology, normal aging
Research Summary:
Our major research interests are the elucidation of inflammatory signaling circuits in Alzheimer’s disease (AD) and in age-related macular degeneration (AMD). AD and AMD represent common, progressive degenerative disorders of human neural (HN) and retinal pigment epithelial (RPE) cells, respectively. Oxidative stress, cytokines, high fat-cholesterol (HF-C) diets, the lipid transporter apolipoprotein E4 (apoE4), and aging, are prominent risk factors for the development of AD and AMD (oval at middle left). These risk factors up-regulate a set of stress-sensitive transcription factors that include, prominently, NF-κB. Promoter mapping of the regulatory regions of the gene encoding beta-amyloid precursor protein (βAPP), is enriched in NF-κB binding sites. Micro RNAs (miRNAs) act as highly effective post-transcriptional repressors of gene expression. NF-κB also up-regulates miRNA-146a expression with resultant down-regulation of sortilin-1 (SORL1) and CFH. SORL1 and CFH down-regulation are associated with increased Aβ peptide generation and Aβ peptide-mediated pathogenic events that (1) contributes to amyloid and drusenoid deposition, (2) enhances inflammatory signaling and apoptosis and (3) drives AD/AMD-type change. In a parallel pathogenic circuit miRNA-29a down-regulation induces up-regulation of beta amyloid cleavage enzyme 1 (βACE1) expression. βACE1-mediated cleavage of the polytopic membrane spanning protein βAPP (green ovals) ultimately increases Aβ peptide abundance that further contributes to amyloid and drusen formation and enhanced inflammatory signaling. Vertical up- or down-arrows within boxes indicate up- or down-regulation, respectively; filled light green box indicates potential blocking compounds – highly penetrant antioxidants such as phenyl butyl nitrone (PBN), the essential omega-3 fatty acid DHA, and miRNA and anti-miRNA strategies. We hypothesize that these specific pathways of genetic mis-regulation in human brain and retinal cells lead to an inflammatory response, resulting in apoptotic changes that are direct precursors to early pathological change in both AD and AMD.
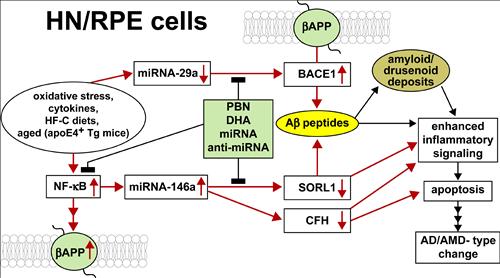
Figure 1: Pathways of interest in our research
http://www.medschool.lsuhsc.edu/faculty/docs/NEUROSCIENCE RETREAT WRITE-UP WJ LUKIW FEB 2009.pdf
Teaching Activities
INTER 132: Biological Systems B
NEURO 203: Investigative Neuroscience
NEURO 250: Molecular Neurobiology
Mentor: Louisiana Biotechnology Research Network (LBRN)
Committees & Administrative Responsibilities
1. Louisiana State University Neuroscience Center Advisory Committee (2004-present)
2. International Travel Committee at LSUHSC-New Orleans (2003-2010)
3. LSU Neuroscience Center Annual Retreat Selection Committee at the Neuroscience Center (1998-present)
4. Graduate Faculty Committee at LSUHSC-New Orleans (2004-present)
5. Faculty Advisory Committee at the LSU Neuroscience Center (1998-present),
6. Graduate Student Selection Committee at the LSU Neuroscience Center (1998-preset),
7. Graduate Affairs Committee (GAC) of the LSU School of Medicine, Louisiana State University -Health Sciences Center (LSUHSC), New Orleans
8. LSU Neuroscience Center Journal Club (1-2 seminars presented per year)
9. LSUHSC Mentors Program (http://www.medschool.lsuhsc.edu/mentors/mentor) in the Department of Ophthalmology, LSUHSC-New Orleans (since 2003), and my area of expertise is: age-related macular degeneration (AMD), Alzheimer’s disease (AD), diabetic retinopathy, neurotoxins in our environment and neurotoxicology.
10. Currently a mentor for, and participant in, the Louisiana Biotechnology Research Network (LBRN); my current mentee is Dr. Perna (Sethi) Dua, Louisiana Technical University, Ruston LA, USA.
Selected Publications
Recent Peer-Reviewed Publications
(selected, last 5 years; from ~315 total)
-
Lukiw WJ,
Pogue AI. Hill JM. SARS-CoV-2 infectivity and neurological targets in the brain; Cellular and Molecular Neurobiology (C&MN) Special Issue: COVID-19 and the Brain in press (2020).
-
Jęśko H, Wencel PL, Wójtowicz S, Strosznajder J, Lukiw WJ, Strosznajder RP. Fingolimod affects transcription of genes encoding enzymes of ceramide metabolism in animal model of Alzheimer's Disease. Mol Neurobiol. 2020 Jun;57(6):2799-2811. doi: 10.1007/s12035-020-01908-3. (2020).
-
Lukiw WJ.
Gastrointestinal (GI) tract microbiome-derived neurotoxins-potent neuro-inflammatory signals from the GI tract via the systemic circulation into the brain.
Front Cell Infect Microbiol.
2020 Feb 12;10:22. doi:10.3389/fcimb.2020.00022. (2020).
-
Cieślik M, Czapski GA, Wójtowicz S, Wieczorek I, Wencel PL, Strosznajder RP, Jaber V, Lukiw WJ, Strosznajder JB. Alterations of transcription of genes coding anti-oxidative and mitochondria-related proteins in amyloid β toxicity: relevance to Alzheimer's disease. Mol Neurobiol. 2020 Mar;57(3):1374-1388. doi:10.1007/s12035-019-01819-y. (2020).
-
Alexandrov PN, Hill JM, Zhao Y, Bond T, Taylor CM, Percy ME, Li W, Lukiw WJ. Aluminum-induced generation of lipopolysaccharide (LPS) from the human gastrointestinal (GI)-tract microbiome-resident Bacteroides fragilis. J Inorg Biochem. 2020 Feb;203:110886. doi: 10.1016/j.jinorgbio.2019.110886. (2020).
-
Lukiw WJ.
microRNA-146a signaling in Alzheimer's disease (AD) and prion disease (PrD). Front Neurol. 2020 Jun 25;11:462. doi: 10.3389/fneur.2020.00462. (2020).
-
Hill JM, Percy ME, Lukiw WJ. Early insight into the potential contribution of aluminum to neurodegeneration - A tribute to the early research work of Robert D. Terry, Igor Klatzo, Henryk M. Wisniewski and Donald R.C. Mclachlan. J Inorg Biochem. 2020 Feb;203:110860. doi: 10.1016/j.jinorgbio.2019110860. (2020).
-
Lukiw WJ,
Pogue AI. Vesicular transport of encapsulated microRNA between glial and neuronal cells.Int J Mol Sci. 2020 Jul 18;21(14):E5078. doi:10.3390/ijms21145078 32708414 (2020).
-
Avsar B, Zhao Y, Li W, Lukiw WJ. Atropa belladonna expresses a microRNA (aba-miRNA-9497) highly homologous to Homo sapiens miRNA-378 (hsa-miRNA-378); both miRNAs target the 3'-untranslated region (3'-UTR) of the mRNA encoding the neurologically relevant, zinc-finger transcription factor ZNF-691.Cell Mol Neurobiol. 2020 Jan;40(1):179-188. doi: 10.1007/s10571-019-00729-w (2020).
-
Lukiw WJ,
Li W, Bond T, Zhao Y. Facilitation of gastrointestinal (GI) tract microbiome-derived lipopolysaccharide (LPS) entry into human neurons by amyloid beta-42 (Aβ42) peptide. Front Cell Neurosci. 2019 Dec 6;13:545. doi:10.3389/fncel.2019.00545. (2019).
-
Zhao Y, Sharfman NM, Jaber VR, Lukiw WJ. Down-regulation of essential synaptic components by GI-tract microbiome-derived lipopolysaccharide (LPS) in LPS-treated human neuronal-glial (HNG) cells in primary culture: relevance to Alzheimer's disease (AD). Front Cell Neurosci. 2019 Jul 10;13:314. doi:10.3389/fncel.2019.00314. (2020).
-
Alexandrov P, Zhao Y, Li W, Lukiw WJ. Lipopolysaccharide-stimulated, NF-kB-, miRNA-146a- and miRNA-155-mediated molecular-genetic communication between the human gastrointestinal tract microbiome and the brain. Folia Neuropathol. 2019; 57(3):211-219. doi: 10.5114/fn.2019.88449. (2019).
-
Jaber VR, Zhao Y, Sharfman NM, Li W, Lukiw WJ. Addressing Alzheimer's disease (AD) neuropathology using anti-microRNA (AM) sStrategies. Mol Neurobiol. 2019 Dec;56(12):8101-8108. doi: 10.1007/s12035-019-1632-0. Jun 10.PMID: 31183807; (2019).
-
Lukiw WJ,
Kruck TPA, Percy ME, Pogue AI, Alexandrov PN, Walsh WJ, Sharfman NM, Jaber VR, Zhao Y, Li W, Bergeron C, Culicchia F, Fang Z, McLachlan DRC. Aluminum in neurological disease - a 36 year multicenter study. J Alzheimers Dis Parkinsonism. 2019;8(6):457. (2020).
-
Zhao Y, Jaber VR, LeBeauf A, Sharfman NM, Lukiw WJ. microRNA-34a (miRNA-34a) mediated down-regulation of the post-synaptic cytoskeletal element SHANK3 in sporadic Alzheimer's disease (AD). Front Neurol. 2019 Feb 6;10:28. doi:10.3389/fneur.2019.00028. (2019).
-
Lukiw WJ,
Cong L, Jaber V, Zhao Y. Microbiome-derived lipopolysaccharide (LPS) selectively inhibits neurofilament light chain (NF-L) gene expression in human neuronal-glial (HNG) cells in primary culture. Front Neurosci. 2018 Dec 5;12:896. doi: 10.3389/fnins.2018.00896. (2018).
- Zhao Y, Lukiw WJ. Bacteroidetes Neurotoxins and Inflammatory Neurodegeneration. Mol Neurobiol. 2018 Dec;55(12):9100-9107. doi:10.1007/s12035-018-1015-y. Epub 2018 Apr 10. PMID: 29637444.
- Alexandrov PN, Pogue AI, Lukiw WJ. Synergism in aluminum and mercury neurotoxicity. Integr Food Nutr Metab. 2018 May;5(3):10.15761/IFNM.1000214. doi:10.15761/IFNM.1000214. Epub 2018 Apr 13. PMID: 29938114; PMCID: PMC6013271.
-
Lukiw WJ, Kruck TPA, Percy ME, Pogue AI, Alexandrov PN, Walsh WJ, Sharfman NM, Jaber VR, Zhao Y, Li W, Bergeron C, Culicchia F, Fang Z, McLachlan DRC. Aluminum in neurological disease - a 36 year multicenter study. J Alzheimers Dis Parkinsonism. 2019;8(6):457. doi: 10.4172/2161-0460.1000457. Epub 2018 Nov 29. PMID: 31179161; PMCID: PMC6550484.
- Hill JM, Percy ME, Lukiw WJ. Early insight into the potential contribution of aluminum to neurodegeneration - A tribute to the research work of Robert D.Terry, Igor Klatzo, Henryk M. Wisniewski and Donald R.C. Mclachlan. J Inorg Biochem. 2020 Feb;203:110860. doi: 10.1016/j.jinorgbio.2019.110860. Epub 2019 Sep 12. PMID: 31698325; PMCID: PMC7012681.
- Alexandrov PN, Hill JM, Zhao Y, Bond T, Taylor CM, Percy ME, Li W, Lukiw WJ. Aluminum-induced generation of lipopolysaccharide (LPS) from the human gastrointestinal (GI)-tract microbiome-resident Bacteroides fragilis. J Inorg Biochem. 2020 Feb;203:110886. doi: 10.1016/j.jinorgbio.2019.110886. Epub 2019 Oct 22. PMID: 31707334.
- Cieślik M, Czapski GA, Wójtowicz S, Wieczorek I, Wencel PL, Strosznajder RP, Jaber V, Lukiw WJ, Strosznajder JB. Alterations of Transcription of Genes Coding Anti-oxidative and Mitochondria-Related Proteins in Amyloid β Toxicity: Relevance to Alzheimer's Disease. Mol Neurobiol. 2020 Mar;57(3):1374-1388. doi: 10.1007/s12035-019-01819-y. Epub 2019 Nov 16. PMID: 31734880; PMCID: PMC7061023.
-
Lukiw WJ. Gastrointestinal (GI) Tract Microbiome-Derived Neurotoxins-Potent Neuro-Inflammatory Signals From the GI Tract via the Systemic Circulation Into the Brain. Front Cell Infect Microbiol. 2020 Feb 12;10:22. doi: 10.3389/fcimb.2020.00022. PMID: 32117799; PMCID: PMC7028696.
- Bihter Avsar, Yuhai Zhao, Wenhong Li, Walter J. Lukiw, Atropa belladonna Expresses a microRNA (aba‑miRNA‑9497) Highly Homologous to Homo sapiens miRNA‑378 (hsa‑miRNA‑378); both miRNAs target the 3′‑Untranslated Region (3′‑UTR) of the mRNA Encoding the Neurologically Relevant, Zinc‑Finger Transcription Factor ZNF‑691.Cellular and Molecular Neurobiology 2019; https://doi.org/10.1007/s10571-019-00729-w
-
Walter J. Lukiw, Lin Cong, Vivian Jaber and Yuhai Zhao. Microbiome-derived lipopolysaccharide (LPS) selectively inhibits neurofilament light chain (NF-L) gene expression in human neuronal-glial (HNG) cells in primary culture, Frontiers in Neuroscience 2018
- Zhao Y, Cong L, Jaber V, Lukiw WJ. Microbiome-derived lipopolysaccharide enriched in the perinuclear region of the Alzheimer's disease brain, Front. Immunol. 2017
-
Wencel PL, Lukiw WJ, Strosznajder JB, Strosznajder RP. Inhibition of Poly(ADP-ribose) Polymerase-1 Enhances Gene Expression of Selected Sirtuins and APP Cleaving Enzymes in Amyloid Beta Cytotoxicity. Mol Neurobiol. 2017 doi: 10.1007/s12035-017-0646-8.
- Alexandrov PN, Percy ME, Lukiw WJ. Chromosome 21-Encoded microRNAs (mRNAs): Impact on Down's Syndrome and Trisomy-21 Linked Disease.
Cell Mol Neurobiol. 2017 Jul 7. doi: 10.1007/s10571-017-0514-0. -
Walter J. Lukiw and Evgeny I Rogaev, Genetics of Aggression in Alzheimer’s Disease (AD) Front. Aging Neurosci. 2017
- Bhattacharjee S, Zhao Y, Dua P, Rogaev EI, Lukiw WJ. microRNA-34a-mediated down-regulation of the microglial-enriched triggering receptor and phagocytosis-sensor TREM2 in age-related macular degeneration PLOS ONE,(2016)
- Pogue AI, Lukiw WJ. Aluminum, the genetic apparatus of the human CNS and Alzheimer’s disease (AD) [Titre Français; Aluminium, appareil génétique du SNC humain et maladie d'Alzheimer], Morphologie,(2016)
- Zhao Y, Alexandrov PN, Lukiw WJ. anti-microRNAs (AMs) as novel therapeutic agents in the clinical management of Alzheimer’s disease (AD). Frontiers in Neuroscience,(2016)
- Clement C, Hill JM, Dua P, Culicchia F, Lukiw WJ. Analysis of RNA from Alzheimer's disease Post-mortem brain tissues. Mol Neurobiol. 53(2):1322-8. doi: 10.1007/s12035-015-9105-6 (2016).
- Zhao Y, Pogue AI, Lukiw WJ. MicroRNA (miRNA) signaling in the human CNS in sporadic Alzheimer's disease (AD)-novel and unique pathological features. Int J Mol Sci. 16:30105-16. doi: 10.3390/ijms161226223 (2015).
- Hill JM, Pogue AI, Lukiw WJ. Pathogenic microRNAs Common to brain and retinal degeneration; recent observations in Alzheimer's disease and age-related macular degeneration. Front Neurol. 6:232. doi: 10.3389/fneur.2015.00232 (2015).
- Hill JM, Lukiw WJ. microRNA (miRNA)-mediated pathogenetic signaling in Alzheimer's disease (AD). Neurochem Res. 2015 Oct 6. PubMed PMID: 26441222 (2015).
- Alexandrov PN, Kruck TP, Lukiw WJ. Nanomolar aluminum induces expression of the inflammatory systemic biomarker C-reactive protein (CRP) in human brain microvessel endothelial cells (hBMECs). J Inorg Biochem. 152:210-3. doi:10.1016/j.jinorgbio. 2015.07.013 (2015).
- Roth W, Hecker D, Fava E. Systems biology approaches to the study of biological networks underlying Alzheimer's disease: Role of miRNAs. Methods Mol Biol. 2016;1303:349-77. doi: 10.1007/978-1-4939-2627-5_21 (2015).
- Zhao Y, Bhattacharjee S, Dua P, Alexandrov PN, Lukiw WJ. microRNA-Based Biomarkers and the Diagnosis of Alzheimer's Disease. Front Neurol. 2015 Jul 13;6:162. doi: 10.3389/fneur.2015.00162 (2015).
- Pogue AI, Dua P, Hill JM, Lukiw WJ. Progressive inflammatory pathology in the retina of aluminum-fed 5xFAD transgenic mice. J Inorg Biochem. 152:206-9. doi: 10.1016/j.jinorgbio.2015.07.009 (2015).
- Pogue AI, Clement C, Hill JM, Lukiw WJ. Evolution of microRNA (miRNA) structure and function in plants and animals: Relevance to aging and disease. J Aging Sci. 2. pii: 119. PubMed PMID: 26146648 (2015).
- Zhao Y, Lukiw WJ. Microbiome-generated amyloid and potential impact on amyloidogenesis in Alzheimer's disease (AD). J Nat Sci. 2015 Jul;1(7). PubMed PMID: 26097896 (2015).
- Zhao Y, Dua P, Lukiw WJ. Microbial sources of amyloid and relevance to amyloidogenesis and Alzheimer's disease (AD). J Alzheimers Dis Parkinsonism. 5:177. PubMed PMID: 25977840 (2015).
- Devier DJ, Lovera JF, Lukiw WJ. Increase in NF-κB-sensitive miRNA-146a and miRNA-155 in multiple sclerosis (MS) and pro-inflammatory neurodegeneration. Front Mol Neurosci. 2015 Mar 2;8:5. doi: 10.3389/fnmol.2015.00005 (2015).
- Hill JM, Lukiw WJ. Microbial-generated amyloids and Alzheimer's disease (AD).Front Aging Neurosci. 2015 Feb 10;7:9. doi: 10.3389/fnagi.2015.00009 (2015).
- Hill JM, Clement C, Zhao Y, Lukiw WJ. Induction of the pro-inflammatory NF-kB-sensitive miRNA-146a by human neurotrophic viruses. Front Microbiol. 3;6:43. doi: 10.3389/fmicb. 2015.00043 (2015).
- Zhao Y, Bhattacharjee S, Jones BM, Hill JM, Clement C, Sambamurti K, Dua P, Lukiw WJ. Beta-amyloid precursor protein (βAPP) processing in Alzheimer's pisease (AD) and age-related macular degeneration (AMD). Mol Neurobiol. 52:533-44. doi:10.1007/s12035-014-8886-3 (2015).
Additional Info
Funding:
"Micro RNA-146a (miRNA-146a) signaling in Alzheimers disease (AD)" -
Investigator - Walter J. Lukiw; Agency NIH - 1R01AG038834-01A1
“Microarray gene expression bi-clustering using associative pattern mining”;
Investigators - Prerna Sethi and Walter J. Lukiw;
Agency - Louisiana Biotechnology Research Network (LBRN).
“Gene expression patterns in glioblastoma multiforme (GBM)”; Investigators - Walter J. Lukiw; Agency - Translational Research Initiative (TRI), Louisiana State University Board of Reagents.
“Mentoring Neuroscience in Louisiana: A biomedical program to enhance neuroscience” (COBRE); Project Director – Nicolas G. Bazan; Mentor – Walter J. Lukiw; Agency - NIH, NCRR
“Rule-based data mining for knowledge discovery in Alzheimer’s disease using Microarray Databases”; Investigators - Prerna Sethi and Walter J. Lukiw; Agency – Louisiana-INBRE program (pending).
"Micro RNA-mediated neurotrophic and synaptic networks in Alzheimer's disease (AD)"; Role on Project: Walter J. Lukiw, Principal Investigator Agency: Alzheimer Association - Investigator-Initiated Research Grant (IIRG), Chicago IL, USA
“miRNA signaling in Alzheimer’s disease (AD)”; Investigator - Walter J. Lukiw; Agency NIH, NIA (pending).

 myLSUHSC
myLSUHSC