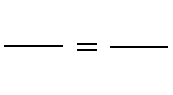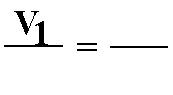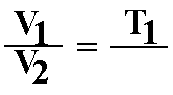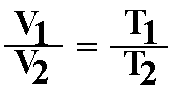
Physical Principles of Gas Exchange
I. Gas Laws
A. Avogadro's hypothesis: Equal volumes of different gases at equal temperature contain the same number of molecules. Equal numbers of molecules in the same volumes at the same temperature will exert the same pressure. (One mole of any gas will contain 6.02x1023 molecules and will occupy a volume of 22.4 L at a temperature of 0°C and a pressure of 760 mmHg.)
B. Dalton's law: In a gas mixture the pressure exerted by each individual gas in a space is independent of the pressures of other gases in the mixture.
e.g. PAlv = PH2O + PO2 + PCO2 + PN2
PGas1 = % of total gases x PTot
C. Boyle's Law: P1V1 = P2V2 (at constant temperature)
D. Charles' law or Gay-Lussac's Law:
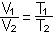
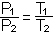
E. Ideal gas Law: PV = nRt; Kinetic theory of gas behavior
F. Henry's Law: The weight of a gas absorbed by a liquid with which it does not combine chemically is directly proportional to the pressure of the gas to which the liquid is exposed (and its solubility in the liquid).
G. Graham's Law: the rate of diffusion of a gas (through the gas phase) is inversely proportional to the square root of its molecular weight.
H. Water vapor pressure: in the body PH2O is dependent only on temperature. At 37OC PH2O = 47 mmHg.
II. Diffusion
B. Diffusion of O2 and CO2 obey Fick's Law:
1. 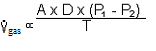 where:
where:
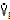 gas = gas flow/time
gas = gas flow/time
A = area
T = thickness
D = diffusivity
P1-P2 = partial pressure gradient
That is, the volume of gas per unit of time moving across a tissue sheet is directly proportional to the surface area of the sheet, the diffusivity, and the difference in gas concentration between the two sides, but is inversely proportional to the tissue thickness.
2. The surface area of the blood-gas barrier is approximately 70 square meters. The thickness is generally 1/2 micron. This large surface area and small thickness are excellent for diffusion.
3. D = Diffusivity or Diffusion constant

CO2 diffuses about 20% slower because of its molecular weight but 24 times faster due to its greater solubility. Therefore the diffusivity of CO2 is about 20 times that of O2.
C. Gas moves through airways by bulk flow until it reaches the terminal bronchioles, after which it moves by diffusion because the linear velocity of bulk flow approaches zero.
D. Exchange between alveoli and capillaries
1. Gas moving from alveolus to blood must pass through:
a. Thin layer of pulmonary surfactant
b. Alveolar epithelium
c. Interstitium
d. Capillary endothelium
e. Plasma
f. Red blood cell membrane g. Red blood cell to reach hemoglobin
2. Time spent by a blood cell in the pulmonary capillaries is about 0.75 to 1.25 sec. at rest.
3. Limitations on O2 transfer (Levitzky Figs 6-1, 6-2):
a. CO-transfer is diffusion limited. Because hemoglobin chemically combines with CO, there can be significant blood CO content at low PCO. Therefore the PCO gradient is well maintained. The limitations on the rate of CO transfer are the diffusion properties of the blood-gas barrier including the area, thickness and diffusivity of CO, since pulmonary blood flow is not a limiting factor.
b. N2O - PN2O rises rapidly as its content in blood is increased. As a consequence the PN2O in the plasma equilibrates with that in alveolar gas in about 1/10 sec. Therefore, after this initial rapid uptake of N2O there is no further transfer of N2O because the partial pressure gradient between the alveolar gas and capillary blood has disappeared. Therefore N2O transfer is perfusion limited.
c. Substantial increase in PO2 as the O2 content increases. Under normal conditions the PO2 in the capillary blood equilibrates with that in alveolar gas after about 1/3 of the time available in the capillary. Thereafter a negligible further transfer of O2 occurs. Under these conditions O2 transfer is perfusion limited.
d. During exercise cardiac output may be greatly increased. The time a blood cell spends in a pulmonary capillary may therefore be reduced to 0.25 sec. and O2 transfer approaches diffusion limitation.
E. Transfer of CO2 (Levitzky Fig. 6-3)
1. Time course of CO2 transfer is similar to that of O2 transfer.
2. Although the diffusivity of CO2 is about 20 x greater than that of O2, the partial pressure gradient is only about 5 mmHg instead of 60 mmHg. Therefore the time course is about the same as for O2.
3. CO2 transfer is normally perfusion limited.
F. Measurement of diffusion: The Diffusing Capacity
Use CO because transfer is diffusion limited.
DLco = 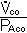
G. Three components of pulmonary diffusion of oxygen or carbon monoxide:
1. Diffusion through the alveolus
2. Diffusion through the alveolar-capillary barrier
3. Uptake by red blood cells.
H. Conditions that decrease the diffusing capacity (Levitzky Table 6-1) include interstitial
or alveolar edema, interstitial or alveolar fibrosis (e.g. sarcoidosis), destruction
of the alveolar-capillary surface (e.g. emphysema), low pulmonary capillary blood
volume, decreased right ventricular output and 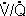 mismatch.
mismatch.
Copyright 2000 M. G. LEVITZKY
Last updated Tuesday, July 23, 2013 12:27 PM
LSUHSC is an equal opportunity educator and employer.
The statements found on this page are for informational purposes only. While every effort is made to ensure that this information is up-to-date and accurate, for official information please consult a printed University publication.
The views and opinions expressed in this page are strictly those of the page author. The contents of this page are not reviewed or approved by LSUHSC.
This page is maintained by webmaster-arc: mgiaim@lsuhsc.edu
