
The Transport of Oxygen and Carbon Dioxide by the Blood
I. Gas transport from ambient air to tissues
A. General scheme (Levitzky Fig. 1-1):
1. Conducting airways - convection or bulk flow
2. Alveoli - diffusion
3. Blood-gas barrier - diffusion
4. Blood - convection or bulk flow
5. Peripheral tissues - diffusion
B. Changes in partial pressures:
1. "Air conditioning"
a. PAtmO2 = 21% (760 mmHg) = 159 mmHg for dry gas. PCO2 = 0.04% (760 mmHg) = 0.3 mmHg
b. Gas in airways (Humidified + warmed) PIO2 = 21% (760-47) mmHg = 150 mmHg PICO2 = 0.04% (760-47) mmHg = 0.3 mmHg
2. Alveolar gas is composed of freshly inspired air (VT of 500 ml - VD of 150 ml = VA of 350 ml) and 2-3 L of gas already in the lungs, much of which has already given up its O2 to the pulmonary capillaries and received CO2. Therefore PAO2 represents both inflow and outflow of O2 and is only about 100 mmHg. PACO2 is about 40 mmHg.
PAO2 = PIO2 - PACO2/R + [F]
3. Expired Air - alveolar air mixed with dead space air:
PÉO2 = 120 mmHg; PÉCO2 = 27 mmHg
II. Transport of O2 by the blood
A. Amount of O2 consumed ( O2) at rest 250-300 ml/min.
B. Physical solution of O2 in the blood
1. 1 ml of plasma at 37C contains 0.00003 ml O2/mmHg PO2. Normal arterial blood with a PO2 of 100 mmHg contains only .003 ml O2/ml or 0.3 ml O2/100 ml blood. Therefore, at a resting O2 of 300 ml/min, C.O. would have to be 100 L/min.
C. Chemical combination of O2 in the blood: Hemoglobin (Hb).
1. O2 carrying capacity of Hb = 1.34 ml O2/gm Hb. Therefore a person with the "normal" 15 gm Hb per 100 ml blood has a Hb O2 carrying capacity of 20.1 ml O2/100 ml blood (100% O2 saturation at 250 mmHg PO2).
2. O2saturation of Hb:
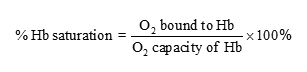
3. The oxyhemoglobin dissociation curve (Levitzky Fig. 7-1):
a. PO2 vs % Hb saturation. Under normal circumstances PO2 of the plasma determines the amount of O2 that combines with Hb.
b. Special properties of the reaction Hb +O2 HbO2.
i. Rapid and reversible: Half time is 0.01 sec or less.
ii. The curve is S-shaped because hemoglobin molecule has four subunits. Therefore each Hb molecule can carry four O2 molecules. There is an interaction of the subunits such that during successive combination with the four O2 molecules each combination facilitates the next. Similarly each dissociation facilitates the next. This S-shape is extremely important physiologically.
iii. The reaction rate is influenced by pH, PCO2 and temperature.
D. Physiologic consequences of the oxyhemoglobin dissociation curve.
1. O2 loading - pulmonary capillaries
a. Relatively flat. This means that Hb will remain highly saturated with O2 (and therefore have a high O content) at fairly low PO2's. (e.g. at a PO2 of 100 mmHg Hb is 97.4% saturated; at a PO of 70 mmHg Hb is still 94.1% saturated). This is of great value to a person at altitude or a patient with respiratory disease. It also means that PO2 reflects the respiratory state better than Hb saturation.
b. pH and PCO2: decreased pH and increased PCO2 can decrease the loading of O2 (the Bohr effect-Levitzky Fig. 7-2A, B). Also increased temperature can decrease O2 loading (Levitzky Fig 7-2C).
c. Other factors interfering with O2 loading (Levitzky Fig. 7-4A):
i. Carbon monoxide - displaces oxygen from hemoglobin
ii. Methemoglobinemia Fe++→Fe+++ (doesn't combine with O2)
iii. Anemia - decreased Hb
2. O2 unloading - tissue capillaries
a. Curve is very steep between 40 and 10 mmHg, which is in the range of metabolically active tissues. (at a PO2 of 40 mmHg, Hb is 75% saturated; At a PO2 of 20 mmHg, Hb is 32% saturated).
b. At metabolically active tissues the pH is lower, the PCO2 is higher, the temperature is higher, and the 2,3 DPG levels (increased in anaerobic metabolism) are higher. These shift the HbO2 dissociation curve to the right and assist in unloading O2. They cause the mixed venous HbO2 dissociation curve to shift to the right (Levitzky Fig. 7-3).
E. Myoglobin: High affinity for O2 - can store O2 (Levitzky Fig. 7-4C).
F. Cyanosis: More than 5 gms deoxy Hb/100 ml arterial blood.
III. Transport of CO2 by the blood:
A. Amount produced at rest: 200-250 ml/min
B. Physical solution:
1. CO2 is 20X more soluble in plasma than is O2. Therefore about 5-10% of total CO2 transported is physically dissolved.
C. Carbamino CO2: About 5-10% of CO2 is transported as carbamino compounds. Deoxyhemoglobin forms more carbamino compounds than oxyhemoglobin.
D. Conversion into bicarbonate (HCO3-): About 80-90% of CO2 is transported as bicarbonate.
E. Role of hemoglobin in CO2 transport:
1. Deoxy Hb is a better H+ acceptor than Oxy Hb. That is, deoxy Hb is a weaker acid.
2. Therefore:
H+Hb + O2 ⇔ H+ + HbO2
3. Therefore deoxy Hb can accept the H+ produced by
CO2 + H2O⇔ H2CO3⇔ H+ + HCO3-, allowing CO2 to be transported as HCO.
4. Oxyhemoglobin is a stronger acid than deoxyhemoglobin, so it allows less CO2 to be transported as bicarbonate. Oxyhemoglobin also forms less carbamino compounds than deoxyhemoglobin.
F. CO2 dissociation curve of whole blood (Levitzky Fig. 7-5):
1. Relatively straight.
2. Oxyhemoglobin shifts curve to the right (Haldane effect).
G. Summary (Levitzky Fig. 7-6).
Copyright 2000 M. G. LEVITZKY
- Last updated Tuesday, JULY 23, 2013 2:47 PM
LSUHSC is an equal opportunity educator and employer.
The statements found on this page are for informational purposes only. While every effort is made to ensure that this information is up-to-date and accurate, for official information please consult a printed University publication.
The views and opinions expressed in this page are strictly those of the page author. The contents of this page are not reviewed or approved by LSUHSC.
This page is maintained by webmaster-arc: mgiaim@lsuhsc.edu
